Thermal and UV burns represent an unmet medical need, particularly in defense or military settings. CBI has a number of humane, validated and optimized superficial, partial thickness and full thickness burn models in rodents and pigs, including mini and Duroc pigs. We have evaluated a number of therapeutic regimes including biologics and stem cell treatments as well as small molecules and immunomodulatory agents in Burns and Dermatitis.
Methods of assessment include:
- Wound healing progression with digital image analysis of wound size and closure
- Draize scoring
- Transepidermal water loss
- Body temperature and body weights, food consumption
- Hematology and clinical chemistry
- Serum and tissue markers of inflammation
- Serial biopsies
- Histopathology and photomicroscopy
- Special stains and immunohistochemistry
- Histomorphometry
- Custom upon request
Complete, timely reporting suitable for regulatory submission (GLP and non-GLP studies).
For more detailed information visit our white paper, download our presentation and our blog.
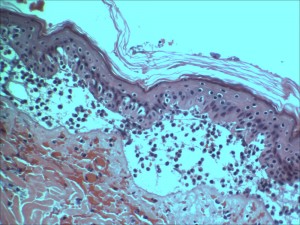
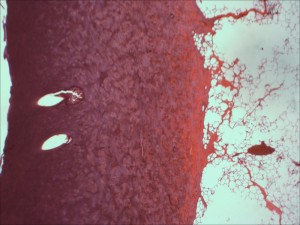
Types of Burns and Dermatitis
- Superficial (1st degree)
- Partial thickness, superficial and deep (2nd degree)
- Full thickness (3rd degree)
- Vertical and progression
- UVB-induced dermal irritation and burns (1st–2nd degree)
Thermal Burn Modeling Therapies
- Topical or systemic administration
- Escharotomy and debridement
- Stem cell therapies
- Autologous cells
- Small molecules
- Biologics
- Bandaging or wound dressings
- Sealants
- Split and full thickness grafting
For more information on our UV burn models, download our presentation.
Thermal Burn Modeling Endpoints
- Wound healing progression
- OCT
- Laser Doppler blood flow
- Draize scoring
- Transepidermal water loss
- Digital image analysis of wound size and closure
- Body temperature
- Body weights
- Hematology and clinical chemistry
- Serum and tissue markers of inflammation
- Serial biopsies
- Histopathology and photomicroscopy
- Special stains and immunohistochemistry
- Histomorphometry
- ELISA
- Custom upon request
- Complete, timely reporting suitable for regulatory submission (GLP and non-GLP studies)



