Neovascular and Vascular Models at CBI
With more than 20 years in the preclinical pharmaceutical and medical device research and development space, CBI offers a wide variety of validated neovascular and vascular models including:
- Laser-induced macular degeneration induction
- Oxygen-induced retinopathy in neonatal models
- STZ-induced retinopathy in diabetic models
- Angiotensin II-induced retinal vascular leakage
- Corneal pocket or suture model
- VEGF-induced retinal vascular leakage
- Laser-induced choroidal neovascularization (CNV) in mini pigs (see below)
For more information on our Retinopathy in Prematurity model, please
download our presentation
Laser-induced Choroidal Neovascularization (CNV) in Mini Pigs
For over 10 years, we have offered established in-house validated Laser-induced Choroidal Neovascularization models for the mouse, rat and rabbit. We are now proud to offer the Laser-induced Choroidal Neovascularization Model in mini pigs. Pigs are favored by the FDA due the similar globe size and retinal anatomy to humans making this model suitable for assessing test article activity in posterior segment proliferative disease.
Using optimized laser settings, uniform, consistent subretinal plaques are reliably produced, additionally, subretinal fibrosis (in some animals) is also seen. Plaque size, lesion intensity, retinal OCT, fluorescein angiography, histopathology and immunohistochemistry may be assessed. Bevacizumab is an appropriate positive control to assess improvements in vascular leakage.
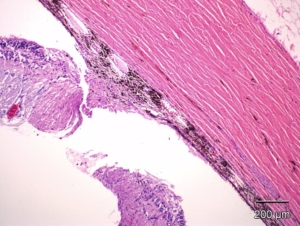
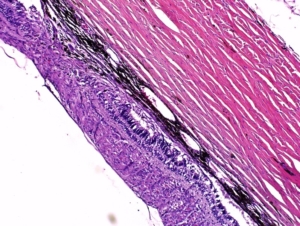
Histopathological Capabilities
Our in-house Histopathology lab offers a wide range of preclinical contract histology and pathology research and development services spanning all aspects of paraffin, frozen and plastic slide preparation, staining and evaluation. Our team of highly trained and experienced board-certified veterinary pathologists, skilled and detail-oriented technical personnel and full-time quality assurance staff are committed to providing the highest level of quality. CBI follows FDA, OECD, EPA, STP, NTP, MHW, FISA and EMEA guidelines suitable for regulatory submission.
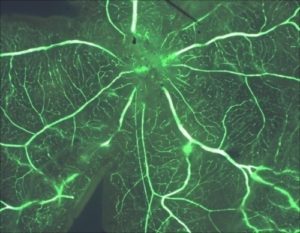
Normal retina from murine species pup.
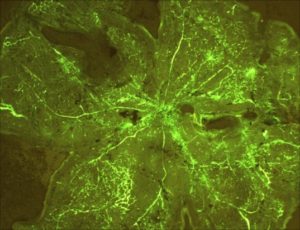
Hyper-oxygenated retina demonstrating vascular proliferation and leakage.