What Is Ototoxicity?
Ototoxicity is when a person develops hearing or balance problems due to a medicine. This can happen when someone is on a high dose of a drug that treats cancer, infections, or other illnesses.
When doctors find ototoxicity early, they may be able to prevent it from getting worse. They also can help kids find the right treatments and therapies to manage problems the condition can cause. Keywords: Ototoxicity, High-frequency audiometry, Distortion product of otoacoustic emission, Ototoxicity monitoring.
DOWNLOAD OUR PRECLINICAL OTIC MODEL PRESENTATION
Auditory Safety and Efficacy
CBI has demonstrated expertise in preclinical contract otic studies — studies relating to the ear. Our highly specialized staff is experienced in providing exploratory and proof-of-concept, and GLP toxicology and pharmacokinetics studies as well as in vivo animal models, pharmacology and histopathology/immunohistochemistry services associated with the outer, middle and inner ear.
We have conducted numerous studies relating to new therapeutics that prevent hearing damage from such concerns as loud sounds, battlefield injuries, ear infections, parasitism, chemotherapeutics, antibiotics, acute and chronic tympanic membrane perforations and surgical manipulations. We have worked with drug delivery systems, which include systemic, local to the ear canal or tympanic membrane or by infusion into the middle or inner ear. Special techniques include peri-lymph collection, cytocochleograms, hair cell analysis, behavioral analysis and auditory brainstem response testing (ABR).
CBI is one of few CRO’s to provide specialty pre-clinical IND enabling GLP or non-GLP Otic and Auditory Toxicology, Efficacy and PK/PD studies in relevant animal species.
Otic Services
- CBI offers otic toxicology, pharmacokinetic and pharmacology capabilities
- Otic modeling and pharmacology
- Acute, subacute and chronic toxicology studies
- Discovery and investigative toxicology studies
- Ototoxic compounds and treatment of ototoxicity
- Ototoxic effects of test articles
- Specialized routes of delivery
- Special otic assessments
- Complete, prompt reports
- Auditory safety
- Otic pharmacokinetics studies
- Otic toxicology studies
- Peri-lymph collection
- Peri-lymph pharmacokinetics
Learn more about our Otic Studies:
AUDITORY ANIMAL MODELS
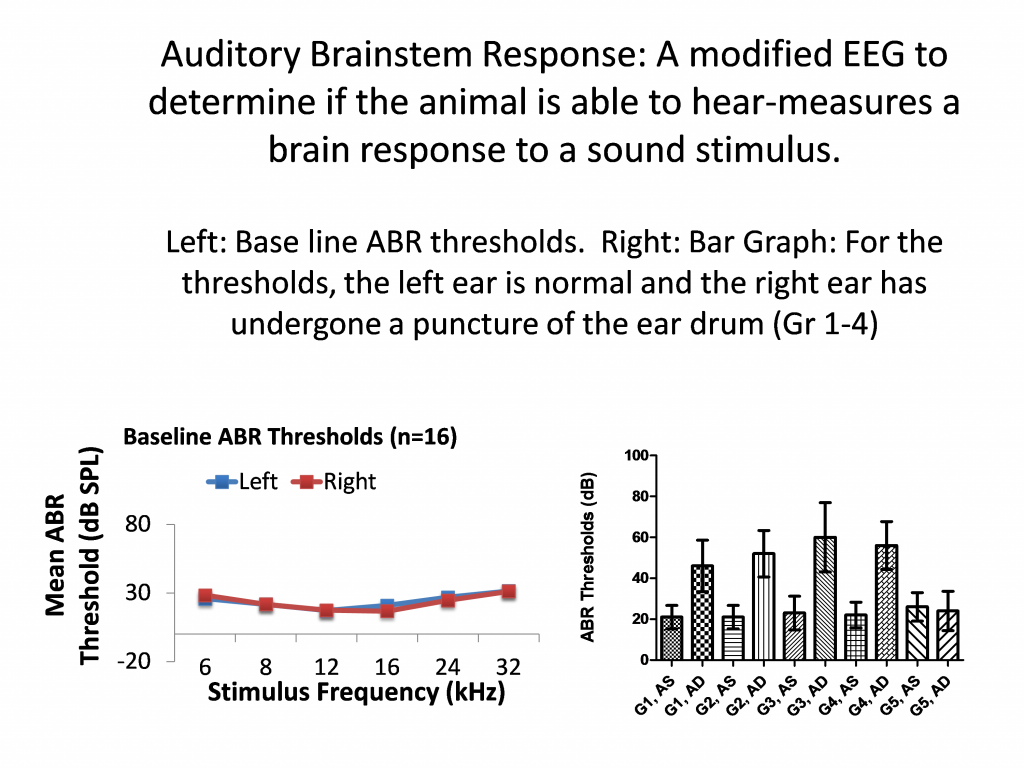
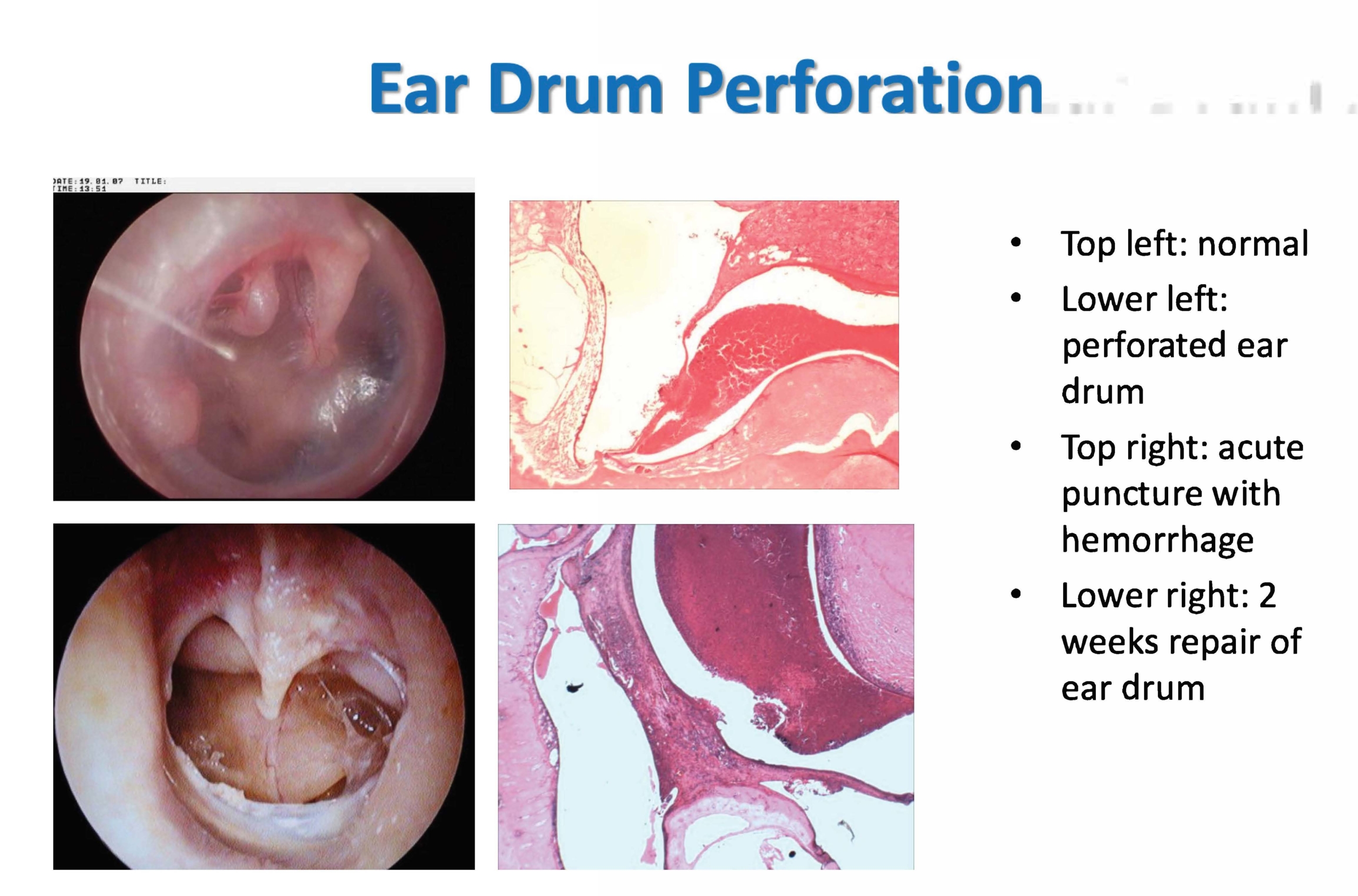
- Ear drum, tympanic membrane perforation
- Bacterial and fungal Infections of external, middle or inner ear
- Aminoglycoside toxicity
- Drug or test article toxicity or effects
- Acoustical hearing loss
- Inflammation or injury to external ear
- Parasitism
- Genetic abnormalities
- Otic devices, delivery devices
- Otic anesthesia
- Unique or customized studies
- FDA driven studies
TOXICOLOGY
- Pilot DRF, acute, and chronic toxicology studies
- Intra-aural, systemic administration and device administration
- Combination toxicology-device, drug, biologic
- PK or TK blood, fluid and perilymph assessment
- Full Histopathology services by ACVP Pathologist
- Unique or customized studies
- FDA driven studies
PHARMACOKINETICS
- Intra-aural and systemic administration
- Otic devices
- Perilymph, blood & fluid monitoring
- Bio-analytical services and method development/transfer
SPECIALIZED TECHNIQUES
- Auditory Brainstem Response Testing
- Hair Cell Assessment
- Cytocochleograms
- Histopathology, Immunohistochemistry, Histomorphometry
- Otoscopy and photography
- Perilymph collection
- Clinical and CNS in life assessments
DRUG DELIVERY CAPABILITIES
- Topical application to ear canal-tympanic membrane
- Inner ear surgical catheterization
- Infusion into middle or inner ear
- Systemic administration
- Device delivery
SPECIES
- Mice and rats
- Guinea Pigs
- Chinchilla
- Cats, dogs, minipigs

Contact Comparative Biosciences, Inc. to discuss a scientific study program for Ototoxicity Studies and Services.
Comparative Biosciences, Inc. · Phone: 408-738-9260
– CUSTOM OTIC STUDIES UPON REQUEST –



