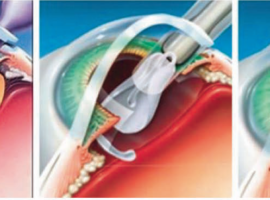
Intraocular Lens Implantation Preclinical Models at CBI
CBI provides a range of sophisticated and customized intraocular implant models. We are offering intraocular lens implantation surgery for cataract treatment particularly in both pigmented and albino rabbits. Our DACVO board-certified ocular… Read More
Histopathology on Human Muscle Biopsies from patients affected with a Glycogen Storage Disease
Provided are thin EPON sections with our unique PAS staining and TEM photomicrographs of patient skeletal muscle with abnormal lysosomes. Glycogen Storage Diseases are devastating, rare genetic orphan diseases for which… Read More
Models of Pulmonary Infection
CBI has optimized and validated four new pulmonary models that have both immune mediated and infection components. Model of Nasal Aspergillosis in Rabbits Chronic invasive fungal rhinosinusitis (Aspergillus fumigatus)may occur… Read More
Cannabis Preclinical Pharmacology and Toxicology
Cannabis Toxicology and Pharmacology. Assessment of Cannabis Products for Purity, Effectiveness and Safety. Now that medical cannabis has achieved some degree of legality and public acceptability,… Read More
CBI Sponsors Ototoxicity and ABRs in Toxicologic Assessments at the ACT
The ACT Annual Meeting is the most cutting edge toxicology meeting anywhere! This year scientists from CBI attended and exhibited at the ACT meeting in Palm Springs CA in November 4-9, 2o17. … Read More
Scleroderma: Tight Skin Mouse Model at CBI
CBI offers preclinical research studies in dermal and pulmonary fibrosis. In addition to our validated bleomycin-induced fibrosis models, we also now offer fibrosis studies with the JAX Tight Skin Mouse Model. There… Read More
Toxicologic and Histopathologic Peer Review
Histopathologic Peer Review (PR) is an important tool in aiding quality and accuracy in the histopathologic assessment of toxicology studies. The peer review verifies and improves the accuracy and quality of pathology… Read More
The (Non)Reproducibility Crisis, Part 4
Harris cites numerous examples of Type I error (also known as false positives) in efficacy studies throughout the book—i.e., drugs that worked in disease models but then failed in human trials. Chapter… Read More
The (Non)Reproducibility Crisis, Part 3
In our previous post, we discussed NPR science journalist Richard Harris’s general critique of animal research in his book Rigor Mortis, but there is more to be said. If animal models are… Read More
The (Non)Reproducibility Crisis, Part 2
In Part 1 of this series, we gave a general introduction to the problem of non-reproducibility of preclinical studies and some reasons why CROs including Comparative Biosciences are somewhat immune to it.… Read More