A validated model for posterior segment proliferative ocular disease at CBI
CBI is known throughout the Industry as a premier ocular preclinical CRO. For over 10 years, we have offered established in-house validated Laser-induced Choroidal Neovascularization models for the mouse, rat and rabbit. We are now proud to offer the Laser-induced Choroidal Neovascularization Model in mini pigs. Pigs are favored by the FDA due the similar globe size and retinal anatomy to humans making this model suitable for assessing test article activity in posterior segment proliferative disease.
Using optimized laser settings, uniform, consistent subretinal plaques are reliably produced, additionally, subretinal fibrosis (in some animals) is also seen. Plaque size, lesion intensity, retinal OCT, fluorescein angiography, histopathology and immunohistochemistry may be assessed. Bevacizumab is an appropriate positive control to assess improvements in vascular leakage.
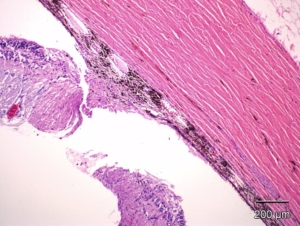
Example of a laser-induced subretinal plaque in minipig. There is disruption of the Bruch’s membrane, edema, inflammation and new vessel formation. HE
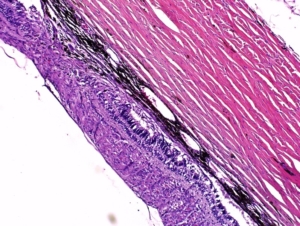
Example of laser-induced subretinal fibrosis with mild exudate and congestion in minipig. There is an area of disruption of the retina and an area of subretinal neovascular plaque formation. HE